
The quest to produce a Bose–Einstein condensate in the laboratory was stimulated by a paper published in 1976 by two Program Directors at the National Science Foundation (William Stwalley and Lewis Nosanow). In 1938, Fritz London proposed the BEC as a mechanism for superfluidity in 4 Einstein proposed that cooling bosonic atoms to a very low temperature would cause them to fall (or "condense") into the lowest accessible quantum state, resulting in a new form of matter. Bosons, particles that include the photon as well as atoms such as helium-4 ( 4 The result of their efforts is the concept of a Bose gas, governed by Bose–Einstein statistics, which describes the statistical distribution of identical particles with integer spin, now called bosons. ) Einstein then extended Bose's ideas to matter in two other papers. (The Einstein manuscript, once believed to be lost, was found in a library at Leiden University in 2005. Einstein was impressed, translated the paper himself from English to German and submitted it for Bose to the Zeitschrift für Physik, which published it in 1924. Right: after further evaporation, leaving a sample of nearly pure condensate.īose first sent a paper to Einstein on the quantum statistics of light quanta (now called photons), in which he derived Planck's quantum radiation law without any reference to classical physics. Center: just after the appearance of the condensate. Left: just before the appearance of a Bose–Einstein condensate. In 2001 Cornell, Wieman and Ketterle shared the Nobel Prize in Physics "for the achievement of Bose-Einstein condensation in dilute gases of alkali atoms, and for early fundamental studies of the properties of the condensates." History Velocity-distribution data (3 views) for a gas of rubidium atoms, confirming the discovery of a new phase of matter, the Bose–Einstein condensate. In 1995, the Bose–Einstein condensate was created by Eric Cornell and Carl Wieman of the University of Colorado Boulder using rubidium atoms later that year, Wolfgang Ketterle of MIT produced a BEC using sodium atoms. This state was first predicted, generally, in 1924–1925 by Albert Einstein, crediting a pioneering paper by Satyendra Nath Bose on the new field now known as quantum statistics.
Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which microscopic quantum mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. In condensed matter physics, a Bose–Einstein condensate ( BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 ☌ or −459.67 ☏).

Although most solutions we encounter are liquid, solutions can also be solid.Schematic Bose–Einstein condensation versus temperature of the energy diagram The specific compositions of both of these solutions are not fixed, however, but depend on both source and location for example, the composition of tap water in Boise, Idaho, is not the same as the composition of tap water in Buffalo, New York.

Thus air is a solution of nitrogen, oxygen, water vapor, carbon dioxide, and several other gases tap water is a solution of small amounts of several substances in water.

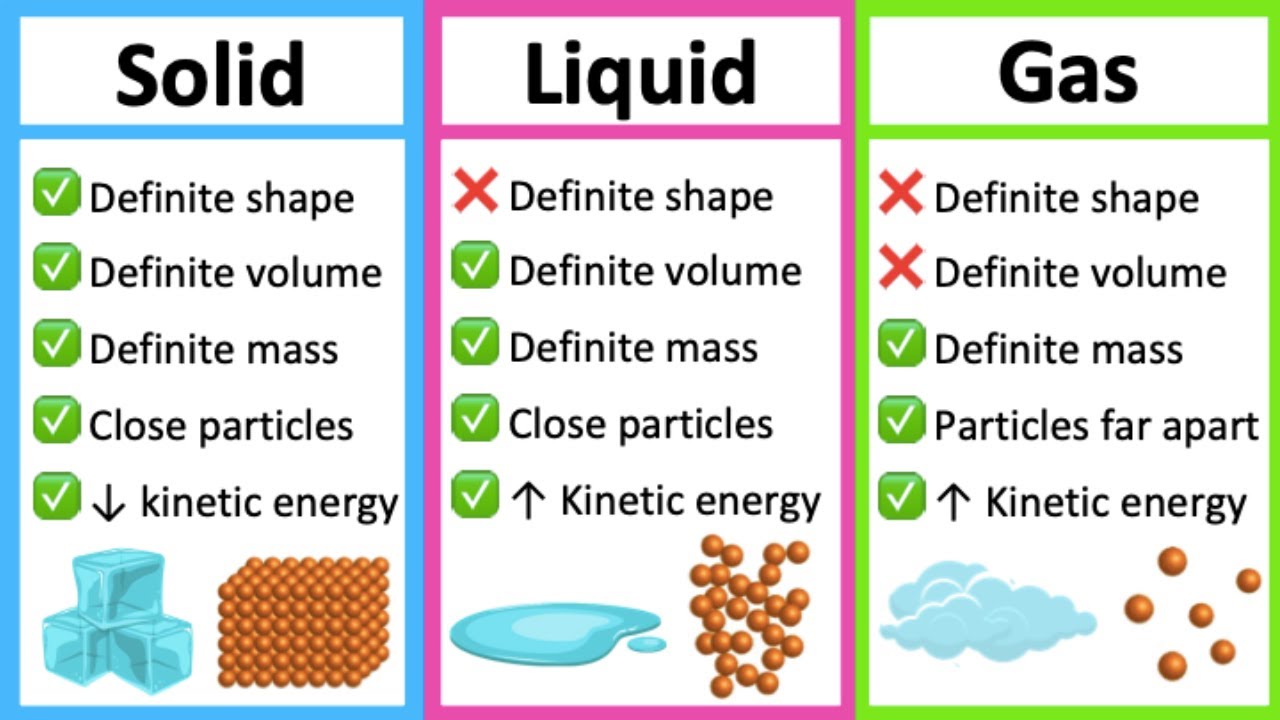
Homogeneous mixtures are also called solutions. Examples of homogeneous mixtures are the air we breathe and the tap water we drink. If all portions of a material are in the same state, have no visible boundaries, and are uniform throughout, then the material is homogeneous. Air, tap water, milk, blue cheese, bread, and dirt are all mixtures. Very few samples of matter consist of pure substances instead, most are mixtures, which are combinations of two or more pure substances in variable proportions in which the individual substances retain their identity. Oxygen, for example, is a pure chemical substance that is a colorless, odorless gas at 25☌. Figure used with permission from WikipediaĪ pure chemical substance is any matter that has a fixed chemical composition and characteristic properties. Gases completely fill their containers, regardless of volume. Liquids have a fixed volume but flow to assume the shape of their containers.


 0 kommentar(er)
0 kommentar(er)
